Calculate the Molar Solubility of Baso4 in Water
A Water flows equally in both directions. E Starch moves out of the 10 starch solution into the 4 starch solution.

The Solubility Of Baso 4 In Water Is 2 33xx10 3 G Litre Its Solubility Product Will Be Youtube
If we let x equal the solubility of Ca 3 PO 4 2 in moles per liter then the change in Ca 2 is once again 3x and the change in PO.
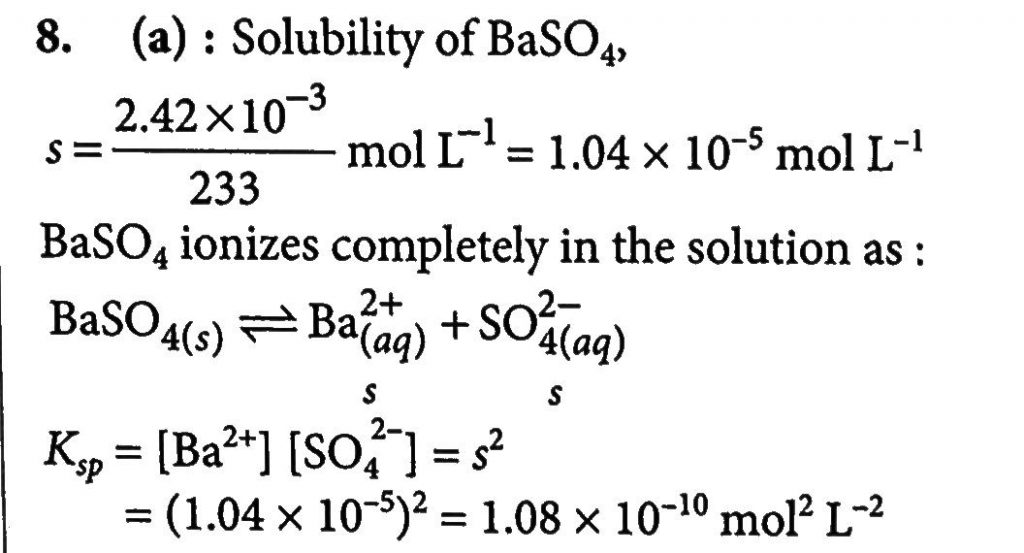
. B There is a net flow of water from the 4 starch solution into the 10 starch solution. D Water does not cross the membrane at all. A precipitation reaction is a process during which two reactants form a precipitate.
C There is a net flow of water from the 10 starch solution into the 4 starch solution. A The balanced equilibrium equation is given in the following table. Calculate the molarity of a solution made by dissolving 500 g of glucose C6H12O6 in sufficient water to form exactly 100 mL of solution.
Discover what precipitates are and learn about the solubility rules net ionic equations and examples of net. Calculate the Molar Solubility of aqueous Barium sulfate at 20 oC in a solution of 00100 M Na2SO4. Substitute the appropriate values into the expression for the solubility product and calculate the solubility of Ca 3 PO 4 2.
500 g C6H12O6 x 1 mol 1802 g 00277 mol C6H12O6 100 ml x 1 L1000 ml 01 L. Ksp BaSO4 11 x 1010. Ksp BaSO4 11 x 1010.
The Solubility Of Baso4 In Water Is 2 42 X 10 3 G L 1 At 298 K Sarthaks Econnect Largest Online Education Community
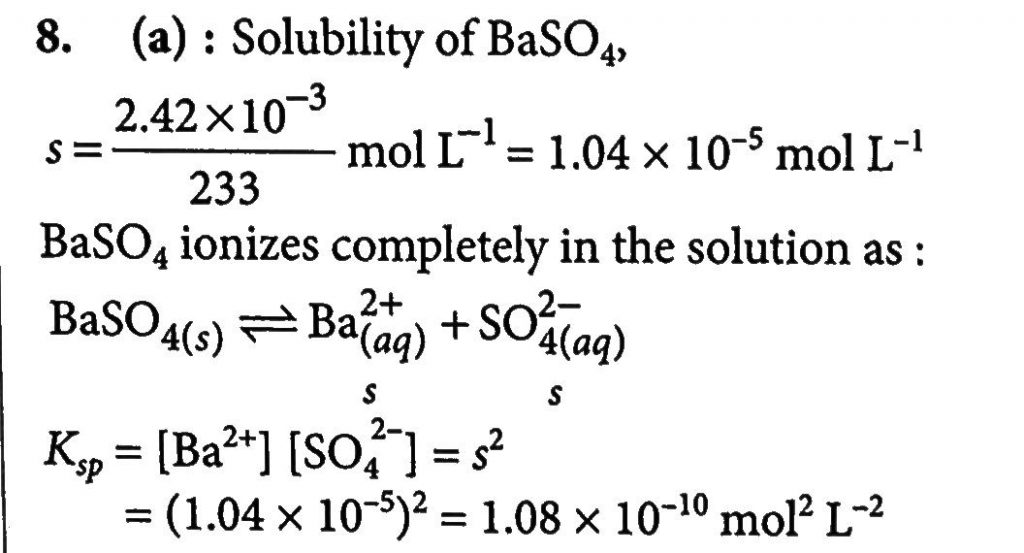
The Solubility Of Baso4 In Water Is 2 42 10 3gl 1 At 298k The Value Of Its Solubility Product Ksp Will Be Given Molar Mass Of Baso4 233gmol 1 Sahay Sir

The Solubility Of Baso4 In Water Is 2 42 10 3 Gl 1 At 298 K The Value Of Its Solubility Product Ksp Will Be Given Molar Mass Of Baso4 233 G Mol 1
No comments for "Calculate the Molar Solubility of Baso4 in Water"
Post a Comment